 |
Don't rush to stuff your electronics into the oven now, but for longer battery life, one day you may be able to bake them when not in use. Over time, the electrodes in the rechargeable battery will grow tiny dendritic filaments, called dendrites or dendrites, which will cause short circuits to scrap the battery and even cause a fire. But thanks to new experiments and computer simulations, researchers at California Institute of Technology (Howard's University of Service, must be very reliable) explored in detail how to decompose these dendrites with higher temperatures - and may extend battery life.
The battery consists of a positive electrode and a negative electrode. When the battery generates current, electrons flow out of the anode, through the external circuit, and then back to the cathode. Some atoms in the anode (consisting of a conductive metal such as lithium) lose the electrons used to generate the current and become ions, and then move to the negative electrode through a conductive liquid medium called an electrolyte.
Recharging the battery reverses this process. The ions return and attach back to the anode, but when they do, the ions do not adhere uniformly. Instead, they form microscopic protrusions that eventually grow into long ones after multiple charge cycles. Branches. When these dendrites arrive and contact the cathode, they cause a short circuit, when the current flows through the dendrites rather than through the external circuit, causing the cell to fail.
The current also heats the dendrites, and because the electrolyte is often flammable, the dendrites may cause a fire. Even if the dendrites do not short-circuit the battery, they can break off from the anode as a whole and float around in the electrolyte, so that the anode loses material and the battery cannot store as much power.
"The dendrites are dangerous and will reduce the capacity of rechargeable batteries," said Asghar Aryanfar, a scientist at Caltech. The new study he led was published this week on the cover of the Journal of Chemical Physics published by the American Physical Society. Although the researchers studied lithium batteries that are the most effective types, their results are widely applicable. "Dendrite problems are common to all rechargeable batteries," he said.
Researchers grew lithium dendrites on test cells and heated them for several days. They found that temperatures as high as 55 degrees Celsius shorten the dendrite by up to 36%. To find out what actually caused this shrinkage, the researchers used a computer to simulate the effect of heat on the individual lithium atoms that make up the dendrites. The model uses a simplified, idealized pyramid geometry.
The simulation results show that the increase in temperature triggers the atom to move in two ways. The atom at the top of the pyramid may fall to a lower height, or the atom energy at a lower height moves and leaves a hole, which is then filled by another atom. These atoms are shuffled to produce enough motion to push down the dendrite.
Aryanfar said that by quantifying how much energy is required to change the dendrite structure, researchers can better understand its structural characteristics. Although there are many factors that affect the battery's lifetime at high temperatures—such as its self-discharge propensity or other chemical reactions that occur in addition—this new study suggests that rejuvenating batteries may require only a little charcoal grilling.
1. Naipu Pump offers all elastomer rubbe wet parts.The high chrome white iron is a very good wear-resistant material and nice wear-resistant is our slurry pumps important character.For the corrosive slurries with blunt particles, we recommend the natural rubber.
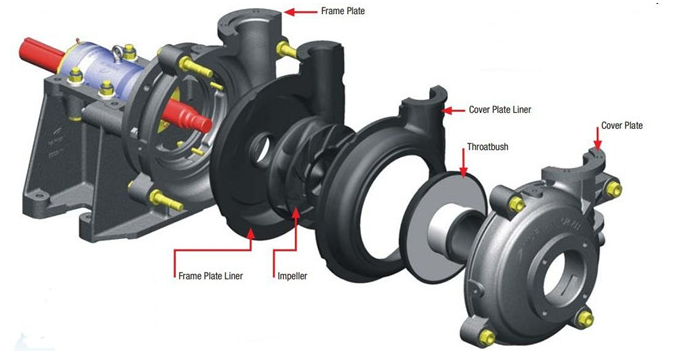
2. Material of Slurry pump wet end parts:
Natural rubber,butyl,neoprene,hypalon,etc.
Slurry Pump Parts,Rubber Slurry Pump Parts,Slurry Pump Rubber Parts,Slurry Pump Impeller
Shijiazhuang Naipu Pump Co., Ltd. , https://www.naipu-pump.com