Solid state batteries are the key technology for the development of next-generation high-security, high-energy density batteries. In the technical route for the development of solid-state batteries, polymer electrolytes have good flexibility, which is conducive to forming good interface contact between electrodes and electrolytes, and can withstand the volumetric deformation of electrode materials during charge and discharge, and have light weight. It is easy to process and suitable for large-scale production, and has attracted wide attention from academic researchers. The traditional preparation process of polymer solid electrolyte (SPE) is usually solution dissolving and pouring-natural air drying film formation-vacuum high temperature drying to remove the solvent. However, because vacuum high temperature drying is a purely physical method, it is difficult to remove 100% of the residual solvent molecules in the SPE film (Figure 1a). The residual liquid will cause the decomposition of the solvent molecules in the battery during the subsequent cycle and the interface with the electrode at the interface. A side reaction occurs, which leads to a series of problems such as increased interface impedance, increased polarization, cycle life and low Coulomb efficiency.
Under the guidance of Researcher Hu Yongsheng and Associate Researcher Suo Liumin, Dr. Liu Lilu and Qi Xingguo, Group E01, Clean Energy Laboratory, Institute of Physics, Chinese Academy of Sciences / National Research Center for Condensed Matter Physics, Beijing, proposed a method for in-situ removal of SPE residues by chemical reaction Free solvent molecule method. The key of this method is to select a suitable solvent, salt and additive combination through control, and skillfully design a two-step chemical reaction process of salt-solvent molecules-additives in the solvent removal process to achieve the final conversion of the residual solvent into a stable additive surface coating (Figure 1b), to achieve the purpose of completely removing residual solvent. Deionized water and NaFSI are used as solvents and salts, respectively, and the polymer is selected to be water-soluble PEO. The SF bond on the NaFSI structure is unstable, and weak hydrolysis occurs in water to produce HF. Further addition of nano-Al2O3 particles converts the intermediate product into AlF3 · xH2O (Figure 1, Figure 2). The SPE prepared by this process effectively reduces the side reaction of the solid-state battery interface, and greatly improves the battery's coulombic efficiency, cycle stability and rate performance.
Assemble solid-state batteries using sodium vanadium phosphate (NVP) and sodium metal (Na) as positive and negative electrodes, respectively. NVP | SPE | Na, NVP | FSI-Al2O3-AQ | Na solid-state batteries have a reversible specific capacity of 110mAh / g in the first week, and Coulomb efficiency 93.8%, which is the level when using liquid electrolyte. During the cycle of NVP | FSI-Al2O3-AQ | Na solid-state battery at 2000 cycles under 1C rate, the Coulomb efficiency is always maintained at ~ 100%. After 2000 cycles, the capacity retention rate is 92.8%, and the average weekly capacity decay rate is only 0.0036%. A symmetrical battery for sodium metal can be circulated stably for 800h at a current density of 100 μA / cm2 (Figure 3b). The electrochemical impedance spectrum also remained relatively stable during the battery cycle. The cyclic stability of the solid sodium battery assembled with the SPE designed in this research work is the best polymer solid sodium battery reported at present (Figure 3).
This work utilizes the salt's water absorption and the nature of the salt itself to achieve in-situ chemical reaction to remove residual solvent (water) molecules in SPE, and the entire preparation process of SPE is carried out in the air, without the need for humidity control or atmosphere protection. At the same time, water as a solvent realizes a green, pollution-free, low-cost SPE preparation process. This work has important reference significance for the development of in-situ reaction control interface and artificial control interface in solid-state lithium / sodium battery. The results of this study were recently published in ACS Energy Letters (ACS Energy Letters, 2019, 4, 1650-1657). The article is titled In Situ Formation of a Stable Interface in Solid-State Batteries. Related work has been supported by the National Key Research and Development Program (2016YFB0901500) and the National Natural Science Foundation of China (51725206, 51421002, and 51822211).
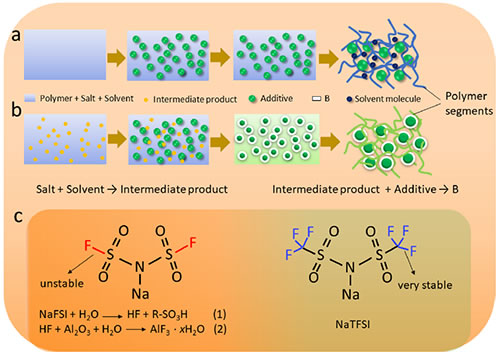
Figure 1. (ab) Schematic diagram of SPE preparation process: a) traditional process; b) designed process; (c) chemical structure of NaFSI and NaTFSI
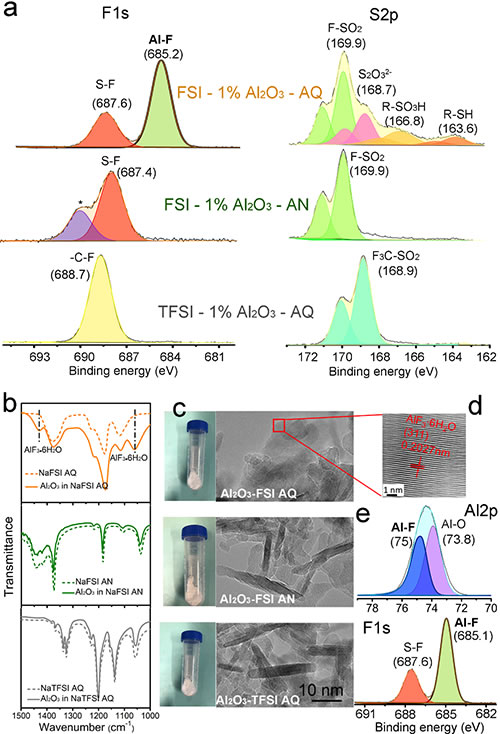
Figure 2. (a) XPS patterns of FSI-1% Al2O3-AQ, FSI-1% Al2O3-AN and TFSI-1% Al2O3-AQ electrolyte membranes; (b) Al2O3 in NaFSI aqueous solution, NaFSI acetonitrile solution and NaTFSI aqueous solution respectively Infrared spectrum after medium reaction; (c) Photographs and TEM images after reaction centrifugation of Al2O3 in NaFSI aqueous solution, NaFSI acetonitrile solution and NaTFSI aqueous solution; (de) High-resolution TEM image after reaction centrifugation of Al2O3 in NaFSI aqueous solution (d ) And XPS map (e)
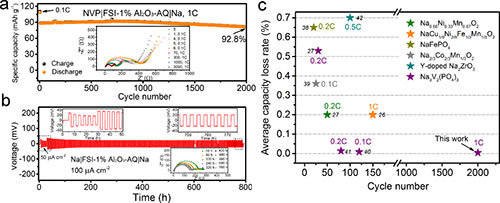
Figure 3. (a) Long-cycle performance of NVP | FSI-Al2O3-AQ | Na and its impedance change during cycling; (b) Cyclic performance of Na | FSI-Al2O3-AQ | Na and its impedance during cycling Change; (c) Summary of average capacity decay rate of polymer solid sodium battery
Gate Valve,Flanged Gate Valve,Threaded Gate Valve,Stainless Steel Gate Valve
Wenzhou Dico Valve Technology Co.,Ltd. , https://www.dicovalves.com