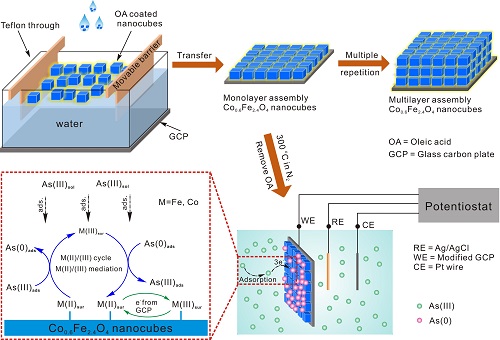
Schematic of Self-assembly of Co0.6Fe2.4O4 Nano-Blocks; Detection of As(III) by Adsorption of Monolayer Nano-blocks and Fe(II)/Fe(III), Co(II)/Co(III) Cycles
The research team of Hefei Intelligent Machinery Research Institute of the Chinese Academy of Sciences, Hefei Institute of Applied Physics, used a Co0.6Fe2.4O4 bulk nanomaterial with a large number of defects on the surface to achieve highly sensitive electrochemical detection of As(III) and enhance its surface defects. The mechanism of electrochemical behavior was studied in detail.
The electrochemical behavior of nanomaterials depends to a large extent on its intrinsic physical and chemical properties, and effectively regulating the structure and electronic state of the nanomaterial surface is of great significance for achieving good electrochemical detection behavior. The large number of defects introduced on the surface of nanomaterials is considered to be an effective method to enhance the performance of nanomaterials. These surface defects can generally serve as active sites to promote the adsorption and catalytic effects of nanomaterials. However, there are few studies on the effect of surface defects on the electrochemical detection behavior. The enhancement mechanism is not yet clear, and the easy-agglomeration of nanomaterials will cause serious masking of active sites of surface defects, resulting in a decrease in the detection performance of nanomaterials. The study of enhancement mechanisms for surface defects poses a number of challenges.
In the earlier work of the research group, the researchers regulated the surface electronic structure of the (001) crystal plane of titania single crystal nanocrystals by doping oxygen vacancies on the surface of TiO2, and improved the nanomaterial pairing by the electrochemical catalysis of oxygen-hole coordination. The electrochemical detection activity of heavy metal ions. Based on this work, the researchers synthesized Co0.6Fe2.4O4 nano-blocks (~14 nm) with a large number of surface defects. In order to maximize the exposure of surface defects, a single layer of nano-particles was dispersed on the electrode by a self-assembly method to construct a sensitive interface for high sensitivity detection of As(III). XPS technology has found that a large number of defects on the surface of nano-blocks serve as active sites for adsorption, which can effectively enhance the adsorption capacity of As(III) on the nano-blocks, thereby increasing the amount of As(III) enriched at the electrode and increasing the electrochemical response. signal. In addition, the presence of defects can increase the Fe(II) and Co(II) activity on the surface of nano-blocks, and make them participate in the redox reaction of As(III) with the intermediate of the active electron-transporting medium. The rate of redox. The effect of cyclic adjustment of Fe(II)/(III) and Co(II)/(III) on detection can be achieved by “As(III) current after the addition of Fe(II) and Co(II) during the detection process. "Increase" is verified. The results of the study indicate that the excellent electrochemical behavior of the nano-blocks is due to the enhancement of surface defects and the mechanism of redox cycle adjustment. The study of the use of nano-materials with large surface defects to enhance electrochemical performance has a guiding significance for constructing a unique sensitive interface for the analysis and detection of heavy metal ions.
The relevant research results are published in the journal Analytical Chemistry. The study was supported by the National Natural Science Foundation of China, the Innovation Crossing Team of the Chinese Academy of Sciences, and the Dean’s Fund of the Hefei Research Institute.
Galvanized Welded Wire Mesh,Welded Wire Mesh Fence,Garden Welded Mesh,Welded Wire Mesh Galvanized
Shenzhou City Hongda Hardware Products Co.,Ltd , https://www.hdpvcwire.com